CellsPlus™
Maximize Your Baby’s Future Health with Advanced Cord Blood Processing Technology
Choosing to bank your baby’s cord blood is a powerful investment in their future. With CellsPlus™, powered by our revolutionary TotiCyte cord blood processing technology, you’re ensuring that this precious resource delivers its maximum potential. Our advanced process is scientifically designed to secure a significantly higher number of viable stem cells, opening doors to more treatment possibilities for your child, now and in the years to come.
The Core Advantage: More Cells for Greater Therapeutic Power
When it comes to cord blood banking, the number of viable stem cells successfully recovered and stored is paramount. This is where CellsPlus™ truly excels.
Treating heavier patients
A bank for life

As your child grows, their body weight increases. Many cellular therapies require a specific dose of stem cells relative to body weight. A cord blood unit that might be sufficient for an infant could be inadequate for that same child as an adolescent or adult. CellsPlus™, by delivering 2.2 to 3 times more stem cells at the point of therapy compared to other common processing technologies, provides a significantly greater chance that the banked sample will be therapeutically effective throughout your child’s life. This “at the point of therapy” count represents the live, functional cells available after thawing for a medical procedure – the number that truly matters.
Access to more treatments
Expanding future options

A larger initial pool of high-quality stem cells, as secured by CellsPlus™, fundamentally broadens the therapeutic horizon. It increases the likelihood that the cord blood unit could be used for multiple medical interventions if ever needed, or for emerging regenerative therapies that may require several doses over time. More cells mean more opportunities to leverage the power of stem cell science.
Enhanced Purity: Optimizing Your Baby’s Stem Cells
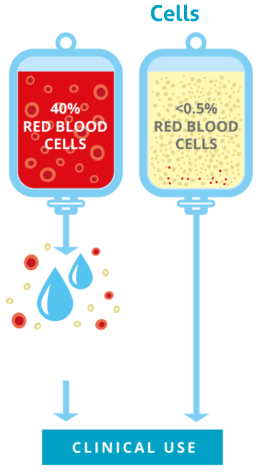
While maximizing cell numbers is primary, the purity of the sample is also beneficial. The CellsPlus™ service, through our TotiCyte™ cord blood processing technology, achieves an exceptional 99% removal of red blood cells. This offers significant upsides:
Improved Blood Type Compatibility
Minimizing red blood cells reduces concerns about blood type mismatches, potentially broadening the unit’s utility for HLA-matched family members.
Preserving Vital Stem Cells
Many cord blood units processed with older methods retain a high percentage of red blood cells (up to 40%), often necessitating an additional “washing” step at the transplant center before infusion. This extra processing can lead to a significant loss of precious stem cells. The superior purity achieved by CellsPlus™ minimizes or often eliminates this need, preserving more of the vital cells for therapy.
Proven Superiority: The Science Behind CellsPlus™
The outstanding performance of CellsPlus™ is not just a claim; it’s backed by rigorous, scientific research. This evidence comes from a study titled “TotiCyte, a Paradigm Shift in Stem Cell Isolation and Storage from Umbilical Cord Blood,” published in a peer-reviewed journal. Peer-reviewed means the study was scrutinized by other experts in the field to ensure its quality and validity before publication.
This crucial study conducted head-to-head tests comparing the TotiCyte™ technology against several other major cord blood processing technology systems widely used in the US, including AXP, HES, MacoPress Smart, and Sepax 2. The publication focused on two key indicators of stem cell quality:
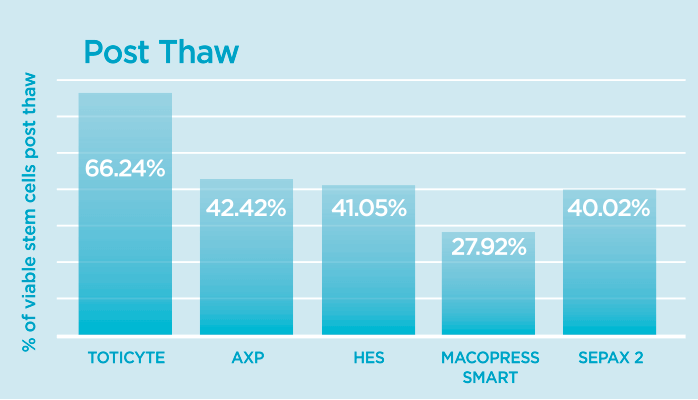
Post-Thaw Viable CD34+ Cell Recovery
This measures the percentage of vital cord blood stem cells (CD34+ cells) that remain after the freezing and thawing process.
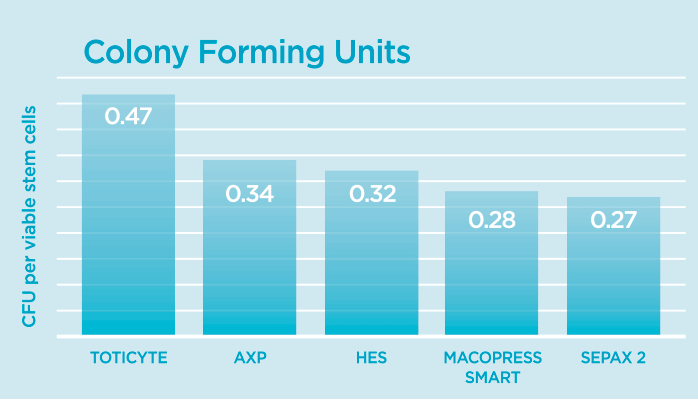
Colony Forming Unit (CFU) Analysis
Considered the gold standard for testing stem cell functionality, this analysis assesses how many viable stem cells retain the ability to grow, divide, and form colonies – a fundamental requirement for successful treatment.
The findings were conclusive: TotiCyte™ technology, which powers our CellsPlus™ service, demonstrated significantly higher post-thaw viable stem cell recovery (retaining 66% of CD34+ cells compared to 38-42% for other systems) and yielded substantially more colony-forming units. This combination of increased cell recovery and potency of the recovered cells resulted in TotiCyte™ performing ~2.2 times better than AXP and 3 times better than MacoPress Smart.
| Processing method | Post-thaw viable CD34+ recovery | CFUs | Overall viable post-thaw CD34+ and CFUs relative to TotiCyte |
|---|---|---|---|
| TotiCyte | 1.00 | 1.00 | 1.00 |
| AXP | 0.64 | 0.72 | 0.46 |
| HES | 0.62 | 0.69 | 0.43 |
| MacoPress Smart | 0.57 | 0.59 | 0.34 |
| Sepax 2 | 0.60 | 0.58 | 0.35 |
CellsPlus™: Designed for Modern Birthing Choices
Delayed Cord Clamping is increasingly common and beneficial for newborns. While it can sometimes result in a smaller cord blood volume for collection, the CellsPlus™ service, powered by TotiCyte™, is expertly designed to maximize stem cell recovery even from smaller sample volumes (e.g., 10-20ml). With CellsPlus™, you can confidently embrace DCC without compromising the quality or quantity of your banked cord blood unit.
Secure Your Child’s Future Health with CellsPlus™
Choosing CellsPlus™ means investing in the most advanced cord blood processing technology available, giving your child the advantage of superior stem cell recovery and purity. This translates to greater potential for future health needs.

