TotiCyte –
a revolution
in cord blood processing
TotiCyte has transformed cell retention both during processing and cryopreservation and is exclusive to Cells4Life.
Found to be the highest performing cord blood processing system when tested in a published, peer-reviewed head-to-head study, TotiCyte delivers 2.2 to 3 times more stem cells at the point of therapy than other leading technologies currently available in the UK.*

Highest cell recovery
TotiCyte delivers two to three times as many viable cells at the point of therapy*
99% red cell removal
TotiCyte removes more red blood cells
Two key factors influence stem cell therapy, the availability of the right number of viable cells and the right type of cell.
Cord blood stem cells are probably the most powerful we will ever have, but the number that can be harvested from the umbilical cord is limited by the amount of blood available.
This is why it is crucial that processing retains as many of your baby’s precious stem cells as possible.
Number of cells matters
TotiCyte delivers more cells when you need them
TotiCyte has been developed to maximise cell count both pre-freeze, and especially post-thaw, as this is the number of stem cells that will be available at the point of treatment. Following a published, peer-reviewed study TotiCyte was found to provide between 2.2 and 3 times more stem cells at the point of treatment relative to other industry leading systems currently available in the UK.
Critically, this means that using TotiCyte ensures that your baby’s sample could be useful throughout their whole life, not just in childhood, and could even be used for multiple therapies.
Your child may be part of the first generation to live to over 100 years of age.
Multiple treatments – your child may be part of the first generation to live to more than 100. Who knows how many times regenerative medicine therapies will be called upon? Each therapy may need to use some or all your baby’s stem cells. Clearly, the more cells stored, the more treatments that can be done. Additionally, with the anticipated development of cell expansion technologies, it will be crucial to have multiple samples available for individual use.
Therapies and body weight – for many therapies, the number of cells required is linked to body weight – as your child grows into an adult, they will need more stem cells for the same treatment.
What does cell recovery mean?
You may see two different types of cell recovery definition used:
Pre-freeze recovery – the number of viable cells that are found after the cord blood has been processed but before it has been cryopreserved.
Post-thaw recovery – the number of viable cells found after the cord blood has been thawed and is ready for therapy. Key point: it is only the number of viable cells that actually matters, dead cells are not useful in regenerative medicine.
Viable cell recovery
TotiCyte has been subject to extensive head-to-head testing against other cord blood processing systems currently available in the UK, Europe and the USA, and was found to perform between 2.2 and 3 times better for viable CD34+ (stem cells) recovery at the point of therapy. These experiments are detailed in a published, peer-reviewed paper, the results of which are summarised below.
The study compared the relative viable cell recovery of CD34+ cells (cord blood stem cells) from cord blood samples processed using TotiCyte (CellsPlus), AXP, HES, the MacroPress Smart and Sepax 2. These are the main systems currently available in the UK, Europe and US.
As you can see, all the methods provide broadly equivalent recoveries of stem cells pre-freeze.
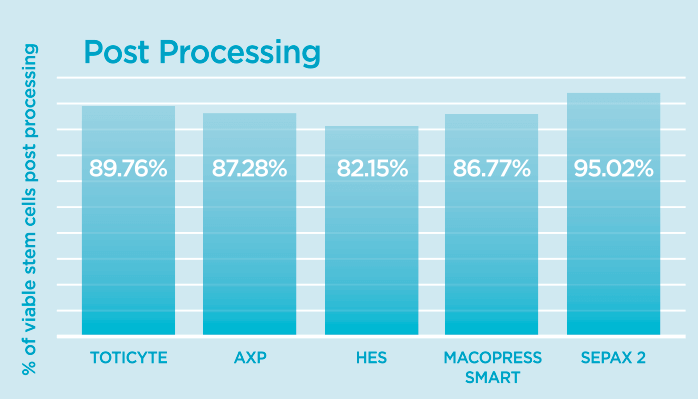
However, the number that really matters is post-thaw, as this is the amount of stem cells that will be left in your baby’s sample after it has been processed, frozen for storage and finally prepared for treatment.
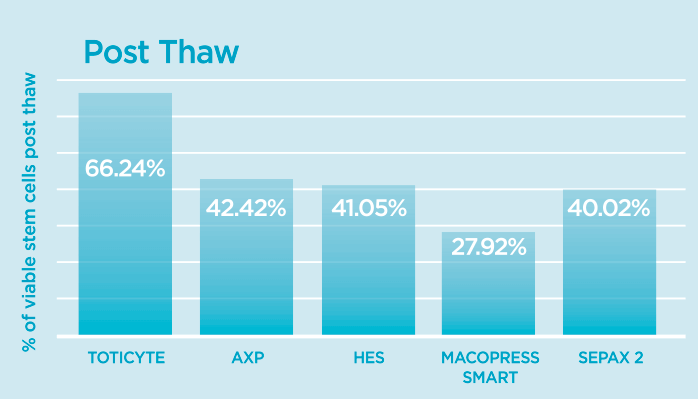
Post-thaw is also where we see the biggest difference. Upon thawing the study found that TotiCyte retained 66% of the CD34+ population of cells, compared to 42% for the AXP, 41% for HES, 38% for MacroPress and 40% for the Sepax 2.
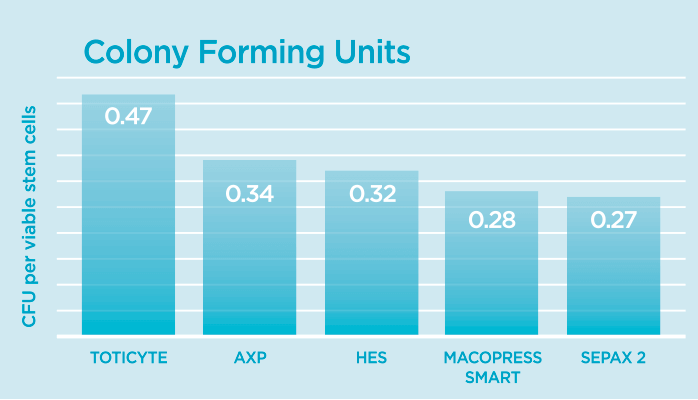
During the study, Colony Forming Unit analysis was carried out. This is the gold standard experiment used to test stem cells before treatment as it tells you of the number of stem cells that appear to be viable, how many have retained the functional ability to grow and divide.
When taking in to account the number of viable stem cells within the thawed samples that had retained the ability to behave like functioning stem cells by forming colonies, TotiCyte retained 2.2 times more viable stem cells than the AXP and 3 times more stem cells than the MacroPress Smart.
Blood type compatibility
If your baby’s cord blood stem cells are used for themselves, they will be a perfect match both at a cellular level and in terms of blood type. However, if they are going to be used to treat another family member, they will have to be checked for compatibility.
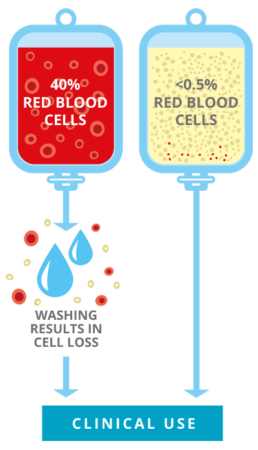
Even for a sibling, where there is a 75% chance of being compatible at a cellular level, it is still possible that their blood types may not match. In this case, the sample may need to be ‘washed’ before treatment to ensure it does not contain too many red cells. This is to reduce the risk of a potentially life-threatening transfusion reaction. The downside of the washing process is that many stem cells are also flushed away, potentially reducing the chance of a successful engraftment and size of person that the sample can treat.
TotiCyte improves blood type compatibility
TotiCyte removes more than 99% of the red cells in cord blood, meaning that it is ready for treatment, no matter the blood type of the patient, and may mean no requirement for washing. This is another way in which TotiCyte delivers the maximum number of stem cells at the point of therapy relative to other industry leading systems.*
Delayed cord clamping – don’t compromise
TotiCyte means that for the first time, cord blood banking is compatible with delayed and optimal cord clamping.
If you choose delayed cord clamping or especially optimal cord clamping, it is likely that there will be less blood available for cord blood collection. For most processing methods, the sample may simply not be large enough to be processed, and even if it is big enough, the cell count may be too low to be therapeutically useful.
TotiCyte can process even the 10-20ml of blood left in the placenta after a long period of delayed cord clamping, and it delivers 3 times more cells at the point of treatment relative to other industry leading systems, meaning even a very small sample can be equivalent to a much larger sample processed using a different method.*
What is TotiCyte?
CE marked and authorised for our use by the Human Tissue Authority, TotiCyte is a precise, low concentration mixture of two solutions routinely used in blood therapy. These are DMSO and Dextran. DMSO and Dextran are present in every single processed cord blood sample and have been transfused into human patients in thousands of cord blood treatments all over the world.

How does it work?
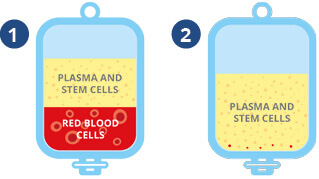
❶ TotiCyte causes the red blood cells to sediment, leaving all other cell types, including the stem cells, suspended in plasma.
❷ The plasma and stem cells are expressed into the next processing bag, leaving 99% of the red cells behind.
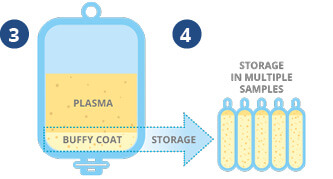
❸ The plasma and stem cells are gently centrifuged, which causes a layer (called the ‘buffy coat’) of stem cells to form at the bottom of the plasma.
❹ The excess plasma is removed leaving a 22ml stem cell sample, which can be divided into multiple units prior to storage.
FIND OUT MORE, REQUEST YOUR WELCOME PACK TODAY
What other techniques are used?
To maintain viability for a future therapy, all public and private cord blood banks will cryopreserve your baby’s cord blood in liquid nitrogen. This will ensure that, whether your baby’s stem cells are needed in 10, 50 or 100 years, they will remain as viable as the day they were harvested.
Processing – Red Cell Depletion
Many different technologies and techniques are used to process and prepare cord blood. The aim of all the leading techniques is to remove as many of the red cells as possible, this is called Red Cell Depletion or Volume Reduction.
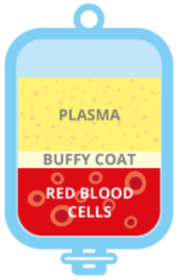
Normally, the cord blood is centrifuged at high speed, which separates the blood into three components: the heavier red cells at the bottom; a very thin layer called the buffy coat which contains the stem cells and; on top, plasma, which contains various factors but no cells.
The buffy coat is then separated into a bag for cryopreservation, along with some plasma and some red cells. Whilst the red cells are not crucial in regenerative medicine, it is important to capture any stem cells that are contained at the interface between the two layers. Similarly, some of the plasma is also taken.
Cryopreservation
The next stage is a two part freezing process, controlled rate freezing to -80℃ followed by placing in vapour phase liquid nitrogen at -190℃.
Immediately prior to controlled rate freezing, about 10% DMSO will be added to the cord blood to help prevent the cells bursting on freezing.
The blood will then remain cryopreserved until needed for a cellular therapy.
Limitations of the other technologies
All Volume Reduction technologies lose stem cells. Post-thaw, many, if not most, viable cells will be lost. These losses are in the range of 70% – 90% of the starting number of viable stem cells. This may limit whether a sample can be used for therapy or how many times it may be used.
In addition, all leading Volume Reduction systems retain as much as 35% red cells, many of which will burst on freezing resulting in cellular debris. This debris can be removed through washing but this will incur the loss of more stem cells. These red cells may also cause a transfusion reaction if the blood types are not compatible.
TotiCyte solves all of these issues, delivering more stem cells and fewer red cells relative to other industry-standard cord blood processing methods.*
Connect with us
*Based on our peer reviewed publication showing that TotiCyte delivers 2.2 to 3 times more haematopoietic stem cells at the point of use than other cord blood processing systems in use in the UK.


