Umbilical cord blood stem cells
Umbilical cord blood stem cells
Umbilical cord blood stem cells
Umbilical cord blood stem cells could change your baby’s life

Powerful

Unique

Safe
Umbilical cord blood is the blood that can be collected from your baby’s umbilical cord and the placenta after they are born. Umbilical cord blood contains billions of powerful stem cells, which are already used to treat over 80 different diseases. They may even hold the key to regenerative medicine. This is why millions of parents in the UK and around the world chose to store blood from their children’s umbilical cord and placenta.
What are stem cells and what can they do?
Stem cells are powerful master cells that have two unique properties:
For example, skin cells act as a protective barrier to the outside world while heart cells pump vital blood around their body. Red blood cells carry the precious oxygen that your child needs to function, and white blood cells form the immune system that prevents them from falling ill.
Given the right conditions and signals, your baby’s umbilical cord blood stem cells can differentiate into these different cell types. As a result, they are extremely valuable in treating an increasing array of medical conditions where specialised cells are damaged and require replacing.
What makes umbilical cord blood stem cells different?
Stem cells are present in many parts of the human body. However, some sources contain richer concentrations than others. Human embryos are also excellent sources of stem cells but their use is controversial and illegal in some countries. In contrast, umbilical cord blood and tissue are collected from material that normally has no use following a child’s birth. They are the richest and purest source of stem cells that your child will ever have.
Storing umbilical cord blood stem cells therefore gives you the opportunity to safeguard your child’s health for years to come. Moreover, they can be easily and safely collected, and reliably stored for treating future health issues. This table compares umbilical cord blood and tissue stem cells with other stem cell sources:
|
|
Cord Blood | Bone Marrow | Peripheral blood | Adipose |
| Potency | ||||
| Availability | ||||
| Routine use in humans | ||||
| Low tumour risk | ||||
| Does not require powerful drugs to collect | ||||
| Contains HSCs | ||||
| Contains MSCs | ||||
| Capacity for high proliferation | ||||
| Low viral contamination risk | ||||
| Non-invasive and painless to collect |
Umbilical cord blood stem cells have the power to repair, regenerate and save lives.
SECURE THIS OPPORTUNITY FOR YOUR UNBORN BABYStem cell types in cord blood
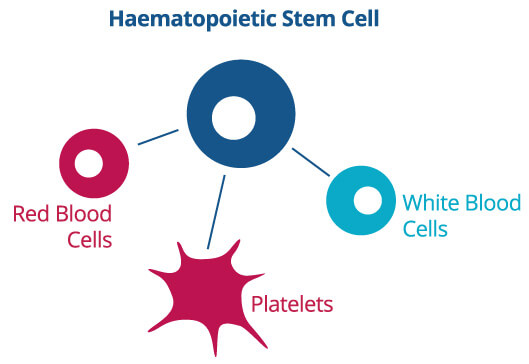
There are two main stem cell types in umbilical cord blood. These are haematopoietic stem cells and mesenchymal stem cells. Each has its own function:
These cells have the ability to transform into various blood cells, including white blood cells. Medical professionals are using this type of stem cell in many current therapies, such as cancer therapy, to repair the immune system.
<<-Click image to enlarge
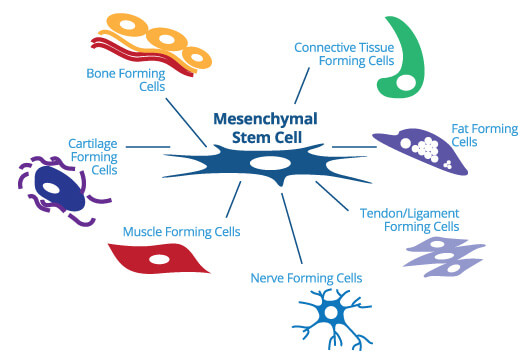
Mesenchymal stem cells (MSCs)
These cells have the ability to transform into a huge range of tissue types, including: nerve tissue / muscle tissue / cartilage.
As a result, these cells are considered extremely important for regenerative therapies. Early studies have shown that mesenchymal stem cells play a crucial role not only in the repair and renewal of these tissue types, but also in regenerating entire organs, such as the heart.
<<-Click image to enlarge
Quick questions…
Evidence Supports Cord Blood Therapy for Cerebral Palsy
A review paper, newly published in the prestigious Pediatrics journal, statistically demonstrates that cord blood therapy for cerebral palsy is an effective treatment to improve the motor skills of children suffering from this condition. The paper is an analysis of...
Cord Blood Cell Therapy Shows Promise in Lymphoma Treatment
A phase 1 trial of a novel cell therapy for lymphoma that is highly resistant to standard treatment (highly refractory lymphoma) has published very positive results in the Nature Medicine journal. The therapy, which uses natural killer (NK) cells derived from cord...
Cord Blood Stem Cell Treatment Offers Superior Results for Knee Osteoarthritis, Study Finds
Osteoarthritis, a chronic degenerative joint disease, affects millions of people worldwide and is a leading cause of disability.[1][2] Currently available treatments can offer relief from pain and other symptoms. However, they fail to address the root of the disease,...
Find out more, request your welcome pack today
Connect with us
* based on our peer reviewed publication showing that TotiCyte delivers 2.2 to 3 times more haematopoietic stem cells at the point of use than other cord blood processing systems in use in the UK.
References
Xiao-Peng Tang et al. “Differentiation of human umbilical cord blood stem cells into hepatocytes in vivo and in vitro” World J Gastronenterology 2006 Jul 7; 12(25):4014-4019 <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4087712/>
Anna Hordyjewska et al. “Characteristics of hematopoietic stem cells of umbilical cord blood” Cytotechnology 2015 May; 67(3):387-396 <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4371573/>
Benedetta Bussolati, “Stem cells for organ repair” Organogenesis 2011 Apr-Jun; 7(2): 95 <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3142444/>
Levitt, Alice. “A Ghost (Heart) of a Chance” Houstonia Magazine, accessed 18 March 2019. <https://www.houstoniamag.com/articles/2017/1/23/texas-heart-institute-new-research-ghost-heart>
N.I. Kalinina et al. (2011) “Mesenchymal Stem Cells in Tissue Growth and Repair” Acta Nature, <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3347612/>
Miao et al. (2017) “A brief review: the therapeutic potential of bone marrow mesenchymal stem cells in myocardial infarction”, Stem Cell Research & Therapy <https://stemcellres.biomedcentral.com/articles/10.1186/s13287-017-0697-9>
Nguyen, L.T., Tran, N.T., Than, U.T.T. et al. Optimization of human umbilical cord blood-derived mesenchymal stem cell isolation and culture methods in serum- and xeno-free conditions. Stem Cell Res Ther 13, 15 (2022). https://doi.org/10.1186/s13287-021-02694-y
Urrutia DN, Caviedes P, Mardones R, Minguell JJ, Vega-Letter AM, et al. Comparative study of the neural differentiation capacity of mesenchymal stromal cells from different tissue sources: An approach for their use in neural regeneration therapies. PLOS ONE. 2019; 14(3): e0213032. https://doi.org/10.1371/journal.pone.0213032
Choi, Y. S., Dusting, G. J., Stubbs, S., Arunothayaraj, S., Han, X. L., Collas, P., Morrison, W. A., & Dilley, R. J. (2010). Differentiation of human adipose-derived stem cells into beating cardiomyocytes. Journal of cellular and molecular medicine, 14(4), 878–889. https://doi.org/10.1111/j.1582-4934.2010.01009.x
Hang H., Yu Y., Wu N., Huang Q., Xia Q., Bian J. Induction of highly functional hepatocytes from human umbilical cord mesenchymal stem cells by HNF4alpha transduction. PLoS One. 2014;9:e104133. doi: 10.1371/journal.pone.0104133
Prabakar K.R., Dominguez-Bendala J., Molano R.D., Pileggi A., Villate S., Ricordi C., Inverardi L. Generation of glucose-responsive, insulin-producing cells from human umbilical cord blood-derived mesenchymal stem cells. Cell Transplant. 2012;21:1321–1339. doi: 10.3727/096368911X612530.
Zhang X., Hirai M., Cantero S., Ciubotariu R., Dobrila L., Hirsh A., Igura K., Satoh H., Yokomi I., Nishimura T., et al. Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells from bone marrow and adipose tissue. J. Cell. Biochem. 2011;112:1206–1218. doi: 10.1002/jcb.23042
Heris, R.M., Shirvaliloo, M., Abbaspour-Aghdam, S. et al. The potential use of mesenchymal stem cells and their exosomes in Parkinson’s disease treatment.Stem Cell Res Ther 13, 371 (2022). https://doi.org/10.1186/s13287-022-03050-4
Li, Z., Dong, X., Tian, M. et al. Stem cell-based therapies for ischemic stroke: a systematic review and meta-analysis of clinical trials. Stem Cell Res Ther 11, 252 (2020). https://doi.org/10.1186/s13287-020-01762-z
Karvelas N, Bennett S, Politis G, Kouris NI, Kole C. Advances in stem cell therapy in Alzheimer’s disease: a comprehensive clinical trial review. Stem Cell Investig. 2022 Feb 21;9:2. doi: 10.21037/sci-2021-063. PMID: 35280344; PMCID: PMC8898169.
Choudhury, T., Mozid, A., Hamshere, S., Yeo, C., Pellaton, C., Arnous, S., Saunders, N., Brookman, P., Jain, A., Locca, D., Archbold, A., Knight, C., Wragg, A., Davies, C., Mills, P., Parmar, M., Rothman, M., Choudry, F., Jones, D. A., Agrawal, S., … Mathur, A. (2017). An exploratory randomized control study of combination cytokine and adult autologous bone marrow progenitor cell administration in patients with ischaemic cardiomyopathy: the REGENERATE-IHD clinical trial. European journal of heart failure, 19(1), 138–147. https://doi.org/10.1002/ejhf.676
Zhou, M., Xi, J., Cheng, Y. et al. Reprogrammed mesenchymal stem cells derived from iPSCs promote bone repair in steroid-associated osteonecrosis of the femoral head. Stem Cell Res Ther 12, 175 (2021). https://doi.org/10.1186/s13287-021-02249-1