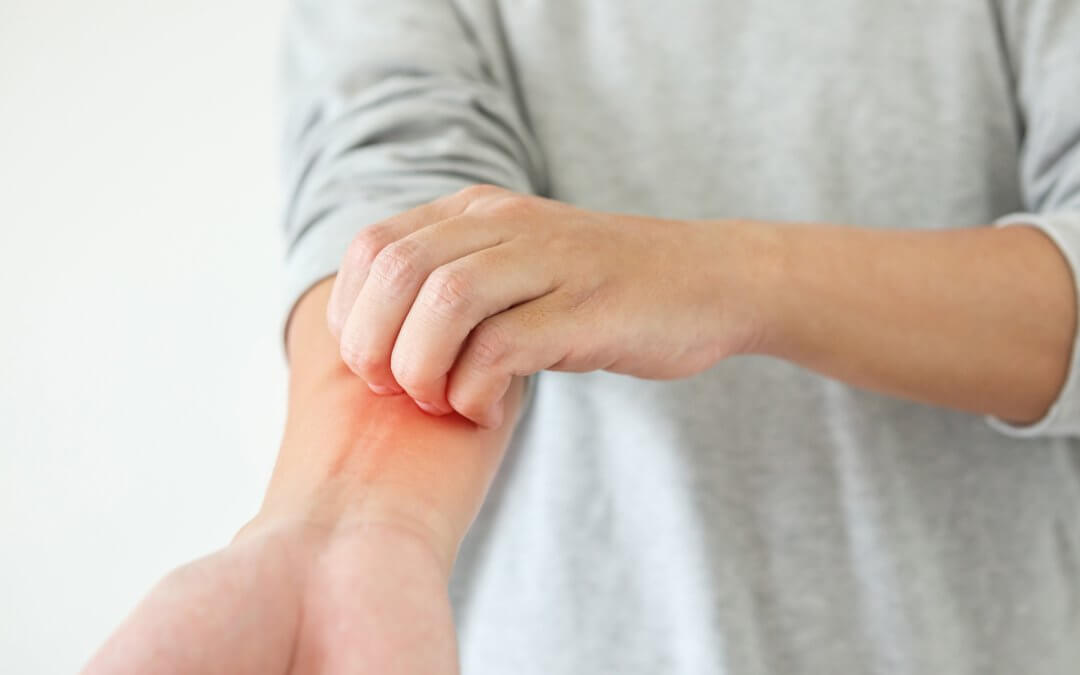
The results from a new study into treating atopic dermatitis (AD) – the most common form of eczema – suggest that mesenchymal stem cells are effective in combatting AD symptoms.
The phase 1/2 trial, which aimed to treat patients suffering from moderate to severe AD symptoms, utilised human bone marrow-derived clonal mesenchymal stem cells (hcMSCs) which were delivered to patients via infusion.
Researchers believe that this treatment method is highly promising in improving symptoms of the chronic condition, which is estimated to affect approximately 2.6% of the population (204 million people) worldwide. [1]
What is atopic dermatitis?
Atopic dermatitis, also known as atopic eczema, is a condition that causes the skin to become dry, cracked, and itchy.
The most common form of eczema in children, atopic dermatitis is a long term condition which can also arise for the first time during adulthood.
The exact causes of AD remain unknown, though it’s thought that it may run in families.
Environmental factors such as the use of certain soaps and cleaning products, the weather, and stress have been shown to be triggers for AD flare ups, and those who suffer from AD often also suffer from hay-fever and asthma.
Current treatment options for AD are limited, with the majority focusing on the alleviation or management of symptoms. Self-care practices, such as avoiding triggers and trying not to scratch, along with the use of moisturisers (emollients) and topical medicines are most commonly recommended to help combat AD symptoms. [2]
In many cases, AD often improves over time, but the current lack of effective treatment options, in addition to the widespread prevalence of the condition and its impact on sufferers’ quality of life, means that finding new ways to combat AD is imperative.
What did the new study find?
The study was conducted in two phases.
Phase 1
- This phase saw 20 patients receive high and low doses of hcMSCs via infusions every two weeks.
- Both high-dose and low-dose application was effective, with 67-70% of both groups displaying a 50% improvement in symptoms.
Phase 2
Phase 2 expanded to 72 patients, half of whom were given the low-dose, and the other half a placebo, with some interesting results:
- 58% of patients who had the low-dose experienced over 50% improvement in symptoms compared to the placebo group, of which only 32% experienced a similar improvement.
- Further yet, 24% of patients who had the low-dose even saw improvements of over 90% in their symptoms thanks to the treatment, compared to only 6% from the placebo group.
Overall, the results of the trial suggest that low doses of hcMSCs can dramatically improve symptoms of AD in sufferers whose condition is moderate to severe. [3]
How does the treatment work?
Mesenchymal stem cells have the ability to regulate the immune system. They interact with various immune cells and help reduce inflammation. Previous studies have shown these cells can lower inflammation in conditions like pancreatitis and improve symptoms in animal models of AD. [4] [5] [6] [7]
In this study, researchers hoped to build upon these previous findings by using mesenchymal stem cells derived from bone marrow in order to help with the immunomodulation necessary for improving AD symptoms.
What’s next for the new AD treatment?
While these results are promising, larger studies are needed to confirm the long-term benefits and safety of this treatment. However, this research opens up an exciting new avenue for eczema treatment that could significantly improve patients’ quality of life.
Cord blood banking and mesenchymal stem cells
The mesenchymal stem cells used in this study come from bone marrow, but they can also be found in a baby’s umbilical cord.
In 2017, a trial showed that mesenchymal stem cells from umbilical cord blood could effectively treat moderate to severe AD. [8]
These mesenchymal stem cells can become various types of specialised cells, from muscle tissue to nerve cells, making them ideal for regenerative therapies.
By saving these stem cells at birth, you may help provide your child with access to potential future treatments, should clinical trials succeed.
To learn more, fill out the form below to receive a free Welcome Pack. This pack will explain how stem cell collection works, the benefits of storing stem cells, and current treatments using stem cells. It only takes a moment, but it could be the best decision you make for your child’s future.
References
[2] (2023, November 11). Overview: Atopic eczema. NHS. https://www.nhs.uk/conditions/atopic-eczema/
[4] Hyun-Min Seo, et al. Phase 1/2 trials of human bone marrow–derived clonal mesenchymal stem cells for treatment of adults with moderate to severe atopic dermatitis, Journal of Allergy and Clinical Immunology,2024,ISSN 0091-6749, https://doi.org/10.1016/j.jaci.2024.06.013. (https://www.sciencedirect.com/science/article/pii/S0091674924006377)
FIND OUT MORE, REQUEST YOUR WELCOME PACK TODAY
All you need to know to make an informed decision.
Provide your contact details to request:
– Complete Welcome Pack and Parent’s Guide
– Information via email
– Contact from our specialist advisors