Perinatal stem cells are those which are collected in and around the perinatal period: the period immediately before and after birth.
These include stem cells from cord blood, cord tissue, the placenta and amnion.
In this blog, we’ll summarise some of their key findings and discuss what they mean in deciding to store stem cells for your baby.
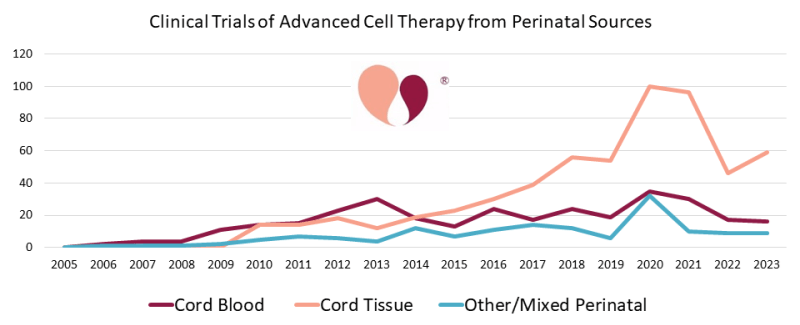
Increase in the number of perinatal stem cell trials, despite COVID-19
In their report, researchers led by Dr Frances Verter point out how clinical trials using umbilical cord mesenchymal stem cells in particular reached a peak in 2020 and 2021, the result of accelerated research to help combat the COVID-19 pandemic.
They estimate that during the pandemic (2020-2022), nearly 41% of all advanced cellular therapy trials for COVID-19 relied on perinatal stem cells.
While the number of trials declined by almost half in the years that followed – to be expected, considering the impact of the pandemic on trial numbers – the researchers found that during the two years since their last report in 2021, the cumulative number of trials for advanced cellular therapies using perinatal stem cells increased by 17%.
This shows that the number of clinical trials using perinatal stem cells has not only returned to pre pandemic levels, but has actually increased, demonstrating a sustained interest in the clinical and therapeutic application of perinatal stem cells.
(graph courtesy of The Parents Guide to Cord Blood Foundation, https://parentsguidecordblood.org/en/news/2024-update-how-many-clinical-trials-employ-perinatal-sources-stem-cells)
Trials for stem cells derived from placenta and amnion
Dr Verter and her team also point out that there are increasingly prominent roles for perinatal stem cells derived from a variety of sources beyond cord blood and cord tissue, namely: placenta and amnion.
Of the 402 clinical trials focusing on the use of mesenchymal stem cells derived or expanded from perinatal tissues, 9% utilised either placenta, amniotic membrane, or a mixture of a number of different perinatal stem cell sources. For reference, only 4% of trials derived MSCs from cord blood.
These numbers suggest that the placenta and amnion are becoming increasingly pivotal to the development of advanced cellular therapies using perinatal sources of stem cells, with the majority of these MSC trials focusing on COVID-19, neurological conditions, orthopaedic conditions and auto-immune disorders.
Cord tissue trials outpace cord blood trials
Since their last report on the cumulative number of trials through to the end of 2021, Dr Verter and her team found that in the years since, through to the end of 2023, there were 117 new trials utilising cord tissue and 40 new trials utilising cord blood. [2]
A key source of mesenchymal stem cells – which have the ability to differentiate into other specialised cell types such as muscle, nerve, and cartilage cells – cord tissue has once again shown itself to be at the forefront of trials utilising perinatal stem cells.
Of the 402 perinatal stem cell trials using MSCs, 87% of these utilised MSCs derived from cord tissue alone.
Considering that during the period from 2019-2023, over 75% of all perinatal stem cell trials were focused on the application of mesenchymal stem cells, cord tissue continues to be a vital source of the kind of stem cells that are driving current therapeutic interest in the application of stem cells from perinatal tissues.
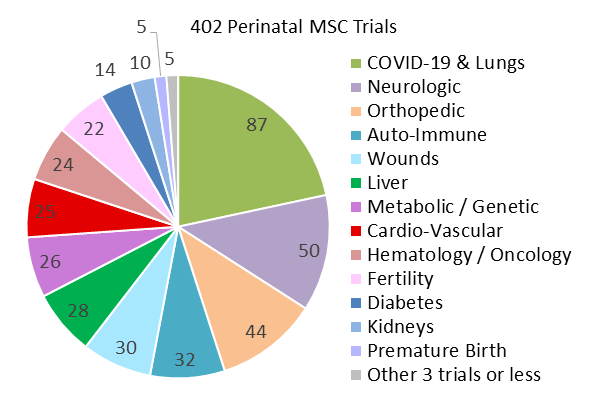
Wide array of medical uses for perinatal mesenchymal stem cells
Another significant finding in this report is the sheer array of diagnosis categories that perinatal mesenchymal stem cells are being trialled for.
While around 45% of trials using perinatal mesenchymal stem cells focused on how they might be applied to treat COVID-19 and other respiratory illnesses, as well as neurological and orthopaedic conditions, another 46% of the total number of perinatal mesenchymal stem cell trials were divided across a wide range of medical diagnoses.
These medical diagnoses spanned trials for auto-immune disorders, the treatment of different forms of wounds, liver conditions, metabolic and genetic conditions (such as Krabbe disease and Hurler’s syndrome), cancers and blood cancers, and cardio-vascular conditions.
These figures suggest not only an increase in the number of perinatal mesenchymal stem cell trials, but also a broad spectrum of possible treatment applications, making them invaluable in the development of future advanced cellular therapies.
(graph courtesy of The Parents Guide to Cord Blood Foundation, https://parentsguidecordblood.org/en/news/2024-update-how-many-clinical-trials-employ-perinatal-sources-stem-cells)
What does this mean for cord blood banking?
If you’re considering storing stem cells for your baby, the findings of this report are encouraging.
The sustained growth in clinical trials using perinatal stem cells, even beyond the pandemic, highlights the continued and expanding interest in their therapeutic potential.
Cord tissue, in particular, stands out as a critical source of mesenchymal stem cells, which are increasingly being used in a wide range of medical applications, from COVID-19 and neurological conditions to auto-immune disorders and orthopaedic treatments.
The growing use of placental and amniotic stem cells further emphasises the value of preserving these additional perinatal tissues, providing a great chance to maximise your baby’s ability to access future treatments.
With ongoing advancements in cellular therapies and the broad spectrum of diseases being targeted, banking stem cells from your baby’s umbilical cord and placenta could offer valuable opportunities for future medical treatments that go beyond what is available today.
Your baby’s stem cells may play a crucial role in the next generation of medical breakthroughs.
If you’re interested in storing stem cells for your baby, fill out the form below to request a free Welcome Pack. It contains information that will help to shed light on the collection process, our storage services, and the future potential offered by umbilical cord stem cells.
References
FIND OUT MORE, REQUEST YOUR WELCOME PACK TODAY
All you need to know to make an informed decision.
Provide your contact details to request:
– Complete Welcome Pack and Parent’s Guide
– Information via email
– Contact from our specialist advisors